Clinical Characteristics, Management and Prognostic Factors of Immune Checkpoint Inhibitor-Associated Pneumonia in Chinese Cancer Patients
By Jinlan Li, Shanshan Chen, Ting Yan, Meizi ZengAffiliations
doi: 10.29271/jcpsp.2024.03.302ABSTRACT
Objective: To investigate the clinical characteristics, treatment methods, outcomes, and variables influencing the outcomes of checkpoint inhibitor-related pneumonitis (CIP) among Chinese cancer patients.
Study Design: Descriptive Study.
Place and Duration of the Study: Department of Pharmacy, Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China, from January 2019 to December 2022.
Methodology: Patients with CIP were inducted. Clinical data including patient characteristics, ICI protocols; and the clinical features, treatments, and outcomes of CIP were collected and analysed.
Results: One hundred and forty-six patients were included. Median time to onset in the CIP was 17.0 weeks (range: 0.4 - 74.7). Mild CIP and severe CIP accounted for 84.93% and 15.07% of cases, respectively. All patients with CIP received methylprednisolone treatment, with an average starting dose of 1.64 mg/kg (0.59-6.00 mg/kg), and 79 (54.11%) of them received anti-infective therapy. One hundred and thirteen (77.04%) patients had improved symptoms of pneumonia, with only 33 (22.60%) patients displaying no improvement. Multivariate analysis revealed that the severity of CIP [OR = 0.167 (95% CI 0.061-0.461), p <0.001] and the starting dose of methylprednisolone [OR = 0.314 (95% CI 0.129-0.764), p <0.001] were independent predictors of outcomes of CIP, while the use of antibiotic was not.
Conclusion: The severity of CIP and the initial dosage of methylprednisolone administered are significant factors that impact the outcomes of CIP in Chinese cancer patients after ICI treatment. Appropriate use of glucocorticoids and antibiotics is a necessary management strategy to control CIP effectively.
Key Words: Immune checkpoint inhibitors, Immune-related adverse events, Checkpoint inhibitor-related pneumonitis, Glucocorticosteroids, Antibiotics, Prognostic factors.
INTRODUCTION
Immune checkpoint inhibitors (ICI), including anti-programmed cell death 1 (PD-1), anti-programmed cell death 1 ligand 1 (PD-L1), and anti-cytotoxic T lymphocyte antigen 4 (CTLA-4) antibodies, might be a significant oncological advancement during the last decade, which has revolutionised the treatment of various types of cancer. Nevertheless, immune-related adverse events (irAEs) may still pose potentially life-threatening risks and impede the applicability of ICIs. One of the most frequently occurring irAEs is checkpoint inhibitor-related pneumonitis (CIP).1
The reported incidence rates of CIP may rise to 5% for all-grade, and around 1% for high-grade pneumonitis.2,3 Although rare, CIP is a severe complication that manifests as dyspnea, cough, fever, chest pain, and pulmonary infiltrates upon chest imaging.4 At present, the treatment for CIP includes glucocorticoid therapy supplemented by immunosuppressive agents, such as infliximab and mycophenolate mofetil. Empiric antibiotics should be considered if an infection has not been completely ruled out.5
Currently, several studies have reported the clinical characteristics and risk factors of CIP, along with its potential effects on tumour prognosis.1,6-8 There are also reports on the effects of glucocorticoids and antibiotics on the prognosis of tumours.9,10 Nonetheless, these studies have included relatively few types of ICIs. Presently, there are 17 ICIs approved for various indications by the National Medical Products Administration (NMPA) as of December 2022,11 and it is conceivable that there might be differences in the incidence of CIP across various ICIs. Moreover, the available research on factors affecting the prognosis of CIP is also relatively scarce.
To develop more personalised and effective strategies for CIP, this retrospective study was conducted to investigate the clinical characteristics, treatment methods, outcomes, and factors affecting the outcomes of CIP in the Chinese population. This study aimed to identify potential factors that may contribute to the therapeutical improvements for CIP.
METHODOLOGY
The present study was a retrospective analysis of patients who received ICI therapy at Hunan Cancer Hospital, between January 2019 and December 2022 and subsequently developed pulmonary inflammatory lesions that were ultimately diagnosed as CIP. The Ethics Committee on Biomedical Research approved this study with partial exemption from informed consent (2023KYKS NO.72).
The inclusion criteria were restricted to patients with cancer, undergoing ICI (PD-1 inhibitors, PD-L1 inhibitors, and/or CTLA-4 inhibitors) therapy, and developing new pulmonary inflammatory lesions after receiving ICI treatment with a definite diagnosis of CIP after the evaluation from the multidisciplinary team. Meanwhile, patients with incomplete or lost follow-up data, and un-blinded patients undergoing randomised controlled trial (RCT) clinical trials for whom immunotherapy had been unspecified were excluded.
The medical records of all eligible patients were comprehensively collected from the hospital’s electronic medical record system. Two types of information were extracted from these records: one for basic information of patients including basic demographic characteristics, underlying diseases, smoking history, histologic type and stage of tumour, lines of ICI treatment, type of ICI and regimen of immune therapy; another information of CIP, such as clinical features including CIP classification, clinical symptoms, radiological manifestations, white blood cell levels, pathogenic culture results, treatments and outcomes of CIP.
The severity of CIP was defined according to the Common Terminology Criteria for Adverse Events, version 5.0 (CTCAE v5.0). CIP is a type of lung parenchyma inflammation, which can be focal or diffuse and is studied through CT imaging. Severity of CIP is classified into four grades: Grade 1 is asymptomatic and confined to one lung lobe or <25% of the lung parenchyma; Grade 2 presents with new or worsening symptoms; Grade 3 is characterised by severe symptoms that involve all lung lobes or >50% of the lung parenchyma, which limits self-care activities of daily living (ADLs) with a required indication for oxygen; and Grade 4 is defined by life-threatening respiratory compromise.
The criteria for the improvement of CIP included the improvement of symptoms, decrease in oxygen requirements, or resolution of radiographic infiltrates. Conversely, worsening was defined as the exacerbation of symptoms, increased oxygen requirements, or progression of radiographic infiltrates.
The data were analysed through the statistical data analysis software IBM SPSS 27.0 (IBM Corp., Armonk, NY, USA). Categorical variables are presented as n (%) while continuous variables are presented as median (range). For continuous variables, the normally distributed data was described by mean and standard deviation (SD), and statistical inference was made by independent t-test. While non-normal ones were described by median and interquartile range (IQR) and inferred by the Wilcoxon rank sum test. Categorical variables were analysed using the chi-square test. Variables with p-values <0.1 in univariate analysis were included in the multivariate logistic regression model. A two-tailed p-value of <0.05 was considered significant.
RESULTS
A total of 146 cancer patients diagnosed with CIP were included in this study. Table I summarises the patient characteristics and immunotherapy protocols. The median time between the first dose of ICI and the onset of CIP was 17.0 weeks, with a broad range from 0.4 to 74.7 weeks. According to the CTCAE v5.0, grades 1 to 4 CIP occurred in 6.16% (9 cases), 78.77% (115 cases), 10.28% (15 cases), and 4.79% (7 cases), respectively. In total, mild CIP (grade 1–2) and severe CIP (grade 3–4) accounted for 84.93% (n = 124) and 15.07% (n = 22) of cases, respectively. The most common presenting symptoms of CIP were cough / sputum (n = 112, 76.71%) and shortness of breath/dyspnea (n = 91, 62.33%). Of these, 15 (10.27%) patients experienced mild to moderate fever. Other less common symptoms included chest pain (n = 2, 1.37%), fatigue (n = 1, 0.68%), hemoptysis (n = 1, 0.68%), nausea and vomiting (n = 1, 0.68%). Additionally, 9 (6.16%) patients showed asymptomatic. All patients showed changes in CT imaging, including chronic obstructive pneumonia-like, interstitial type, ground-glass opacity, and pneumonitis not otherwise specified.
The mean white blood cell count before treatment was 8.22 *109/L (range 2.27-25.18 *109/L). Among these patients, 129 (88.36%) cases had negative pathogenic cultures, or no pathogenic test results, while 17 (11.64%) cases tested positive for pathogens, including Staphylococcus aureus (n=5, 3.42%), Klebsiella pneumoniae (n=4, 2.74%), Streptococcus viridans (n=2, 1.37%), Serratia marcescens (n=2, 1.37%) and other pathogens (n=4, 2.74%, Figure 1).
Table II displays the glucocorticosteroids treatment, concurrent infection and antibiotic treatment, as well as the resulting outcome of CIP. Among the 146 patients with CIP, 113 (77.04%) patients demonstrated improved pneumonia symptoms, while 33 (22.60%) showed no improvement. The starting dosage of glucocorticoids shows a significant difference between the CIP-improved and unimproved groups, while there is no difference in post-treatment white blood cell (p-WBC), organisms isolated, and antibiotic usage between the two groups.
The multivariate analysis revealed significant differences in the grade of CIP [p <0.001, OR = 0.167 (95% CI 0.061-0.461)] as well as in the starting dose of methylprednisolone [p = 0.011, OR = 0.314 (95% CI 0.129-0.764)] between clinical response groups, whereas other factors (including age, gender, underlying diseases, stage of tumour, line of ICI treatment, antibiotics) were not statistically significant.
Table I: Patient characteristics and immunotherapy protocols (n = 146).
|
Characteristics |
Varieties |
No. |
Frequency (%) |
|
Age, years |
Median (range) |
61 (17-81) |
|
|
<65 |
86 |
58.9 |
|
|
≥65 |
60 |
41.1 |
|
|
Gender |
Female |
21 |
14.4 |
|
Male |
125 |
85.6 |
|
|
Underlying diseases |
Cardiovascular disease |
26 |
17.8 |
|
Chronic pulmonary disease |
11 |
7.5 |
|
|
Diabetes |
8 |
5.5 |
|
|
Diabetes+Cardiovascular disease |
6 |
4.1 |
|
|
Chronic kidney disease |
1 |
0.7 |
|
|
Smoking history |
Yes |
119 |
81.5 |
|
No |
27 |
18.5 |
|
|
Histologic type |
Lung cancer |
126 |
86.3 |
|
Adenocarcinoma |
38 |
26.0 |
|
|
Squamous |
61 |
41.8 |
|
|
Small cell |
22 |
15.1 |
|
|
Large cell |
5 |
3.4 |
|
|
Othera |
20 |
13.7 |
|
|
Stage of tumour |
Ⅱ |
2 |
1.4 |
|
Ⅲ |
28 |
19.2 |
|
|
Ⅳ |
116 |
79.5 |
|
|
Line of ICI treatment |
Neoadjuvant |
5 |
3.4 |
|
1st |
104 |
71.2 |
|
|
2nd line |
25 |
17.1 |
|
|
≥3rd line |
12 |
8.2 |
|
|
Treatment regimen |
Pembrolizumab |
41 |
28.1 |
|
Camrelizumab |
28 |
19.2 |
|
|
Sintilimab |
24 |
16.4 |
|
|
Toripalimab |
13 |
8.9 |
|
|
Durvalumab |
9 |
6.2 |
|
|
Atezolizumab |
8 |
5.5 |
|
|
Nivolumab |
6 |
4.1 |
|
|
Otherb |
17 |
11.6 |
|
|
Regimen of immune therapy |
Monotherapy |
11 |
7.5 |
|
Combination therapy |
135 |
92.5 |
|
|
ICI + Chemotherapy |
122 |
83.6 |
|
|
ICI + Antiangiogenesisc |
13 |
8.9 |
|
|
a Head and neck cancer (9 cases), Esophageal cancer (5cases), Gastric cancer (3 cases), Melanoma (3 cases). b Sugemalimab (5cases), Tislelizumab (4cases), Cadonilimab (3cases), Penpulimab (3cases), Serplulimab (2cases). c Bevacizumab (5cases), Anlotinib (5cases), Apatinib (3cases). |
|||
Table II: Glucocorticosteroids (GCS), antibiotics treatment and outcomes of CIP.
|
Variable |
Total |
Improved |
No improved |
p-value |
|
Starting dose of Methylprednisolone (mg/kg), median (IQR) |
1.60 (1.09, 2.0) |
1.52 (1.05, 1.88) |
1.97 (1.60, 2.28) |
<0.001d |
|
<2 mg/kg |
109 (74.66%) |
92 (63.01%) |
17 (11.65%) |
<0.001e |
|
≥2 mg/kg |
37 (25.34%) |
21 (14.38%) |
16 (10.96%) |
|
|
No infection therapy |
67 (45.89%) |
56 (38.36%) |
11 (7.53%) |
0.100e |
|
Infection therapy |
79 (54.11%) |
57 (50.44%) |
22 (66.67%) |
|
|
Target therapy |
17 (11.64%) |
11 (9.73%) |
6 (18.18%) |
|
|
Empiric therapy |
62 (42.47%) |
46 (40.71%) |
16 (48.49%) |
|
|
Antibiotics |
79 (54.11%) |
57 (50.44%) |
22 (66.67%) |
|
|
Cefperazone/Sulbactam |
26 (7.81%) |
19 (16.81%) |
7 (21.21%) |
|
|
Piperacillin/Tazobactam |
14 (9.59%) |
10 (6.85%) |
4 (2.74%) |
|
|
Fluoroquinolonesa |
11 (7.53%) |
7 (4.79%) |
4 (2.74%) |
|
|
Carbapenemsb |
12 (8.22%) |
6 (4.11%) |
6 (4.11%) |
|
|
Cephalosporins |
3 (2.05%) |
3 (2.05%) |
0 (0.00%) |
|
|
Combination therapyc |
13 (8.90%) |
12 (8.22%) |
1 (0.68%) |
|
|
Antibiotics course (days), median (IQR) |
7.0 (5.0, 10.0) |
8.0 (5.0, 11.0) |
7.0 (4.5, 9.25) |
0.170d |
|
a Fluoroquinolones: levofloxacin (10 cases), moxifloxacin (1 cases); b Meropenem (11cases), imipenem (1case); c Piperacillin/Tazobactam + fluconazole (5 cases), Cefperazone/Sulbactam + levofloxacin (3 cases), Ceftriaxone + levofloxacin (2 cases), Meropenem + Linezolid (1 cases), Piperacillin/Tazobactam + Vancomycin (1 cases), Piperacillin/Tazobactam + moxifloxacin (1 cases). d Statistical analysis: Wilcoxon rank sum test. e Statistical analysis: Chi-square test. IQR: interquartile range. |
||||
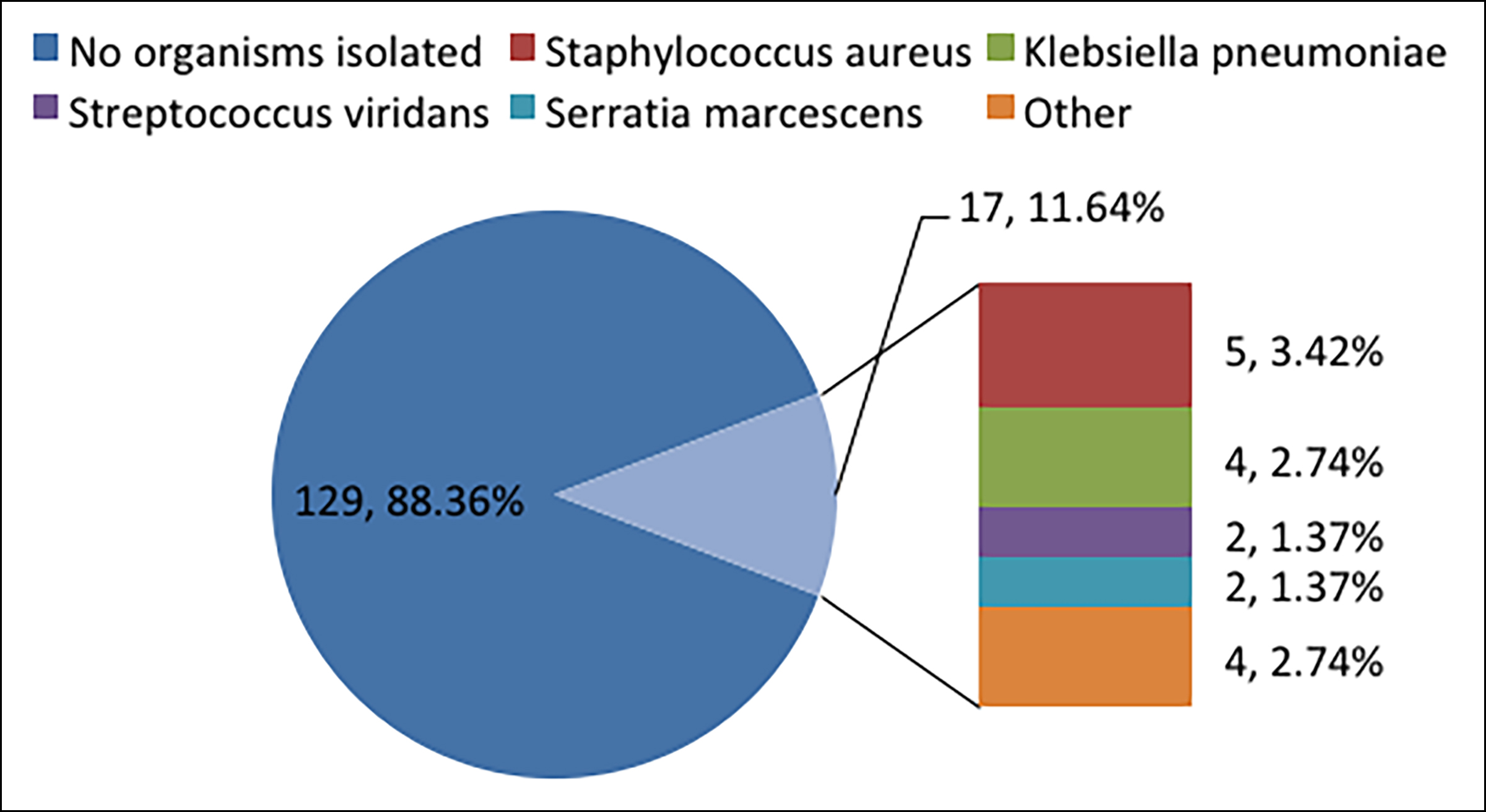 Figure 1: The pathogen culture results of 146 patients.
Figure 1: The pathogen culture results of 146 patients.
Other, Acinetobacter baumannii (1case), Haemophilus influenza (1case), enterobacter cloacae (1case), stenotrophomonas maltophilia (1case).
DISCUSSION
Early identification and standardised treatment management of CIP are crucial in light of their rising prevalence. Such management requires the participation of a multidisciplinary team, including oncologists, laboratory personnel, pharmacists, and nursing staff. To the best of this knowledge, this is the first study to investigate treatment methods, outcomes, and factors affecting the outcomes of CIP among a cohort of Chinese cancer patients treated with ICI. The severity of CIP [p <0.001, OR = 0.167 (95% CI 0.061-0.461)] and initial dose of methylprednisolone [p = 0.011, OR = 0.314 (95% CI 0.129-0.764)] were significant factors influencing the outcomes of CIP, whereas other factors were not statistically significant.
Currently, glucocorticoids remain the primary treatment method for most cases of CIP and early use of gluco-corticoids is crucial for managing immune-related toxicities comprehensively.12-14 It is recommended to interrupt ICI treatment and start low-dose steroids with 0.5 to 1 mg/kg/d if patients with grade 1 CIP deteriorated.15 Grade 2 CIP patients might benefit from withholding ICIs and commencing with intermediate-dose steroids with 1 to 2 mg/kg/d, followed by gradually reducing the dose by 5 to 10 mg every week during 4 to 6 weeks, according to certain proposals.12 Patients exhibiting grade 3 to 4 CIP must terminate ICI therapy immediately and permanently. The American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) guidelines have approved the application of initial steroids doses set at 1 to 2 mg/kg/d and 2 to 4 mg/kg/d, respectively.13,14 In this cohort, CIP patients were all treated with methyl-prednisolone. For the majority of patients (74.66%), the initial dose of glucocorticoid (GSC) was 1-2 mg/kg, while for a minority of patients (25.34%), the initial dose was greater than 2 mg/kg. An initial dose of 6 mg/kg was given to one patient that did not match the recommended guidelines. For patients with grade 3-4 CIP, if symptoms did not improve within 48 hours after glucocorticoids treatment, combined immunosuppressant therapy, such as TNF-α inhibitors (infliximab), mycophenolate mofetil or intravenous immuno-globulin, should be considered instead of increasing the dose of glucocorticoids, since high doses of glucocorticoids may increase the risk of infection, gastrointestinal injury, and osteoporosis.12
The infectious complications were increasingly accompanied with the use of ICIs. Additionally, the use of glucocorticoids is a well-known risk factor associated with infectious complications. In the real world, the incidence of severe infections resulting from ICI administration in patients with melanoma, renal cell carcinoma, and non-small cell lung cancer reached 14% and bacterial infections were found to be the most common cause of infection following ICI treatment.16 Additionally, data on infections in patients receiving treatment for ICI-associated irAEs are scarce. This study focused on examining the extent of infections and the use of antibiotics among CIP patients, which demonstrates a notable departure from the previous study’s objectives. The study revealed a low prevalence of pathogenic bacterial infection among CIP patients, with only 17% testing positive, and culture results showed that common pathogenic bacteria causing pneumonia were found. Additionally, most patients were not tested for infection-related indicators such as procalcitonin (PCT) and C-reactive protein (CRP). Consequently, 79% of CIP patients undergo empirical anti-infection treatment.
Presently, most research focuses on the impact of antibio-tics on tumour prognosis. Nonetheless, limited investi-gations have been carried out to explore the reasons behind utilising antibiotics for patients with CIP and the potential influence of antibiotics on CIP outcomes. The use of antibiotics has been linked to a negative cancer outcome, as reported by previous studies.17,18 A new study has indicated that administering antibiotic therapy before the initiation of ICI therapy, but not simultaneously, was associated with an inferior treatment response and overall survival.19 Hence, prudent use of antimicrobials should be encouraged, considering both the clinical symptoms and the risk of infection. This research indicated that 77.04% of CIP patients showed improvement in pulmonary symptoms, but the use of antibiotics was not a factor affecting CIP recovery. The severity of CIP, alongside the dosage of glucocorticoids, proved to be the more significant factors that impact recovery rates. Of the 79 individuals who received antibiotics within the study, 62 were given empirically, without any microbial evidence, and there was a lack of evaluation of infection indicators such as WBC, PCT, and CRP. As a result, the authors recommend that, before the administration of antibiotics, the use of antibiotics should be evaluated based on the patient's infection symptoms and infection indicators, with a more rational approach to their usage.
There were several limitations to this study. First, it was a single-centric retrospective study with a small sample size, which increases the risk of patient selection bias and group selection bias. Larger studies will be required to verify these results. A second limitation is that the definition of pneumonia and ICI-induced pneumonitis can pose challenges, particularly with the absence of a uniform approach to the diagnostic evaluation of pulmonary symptoms and signs. Third, due to the short hospitalisation time of the patient, combined with the doctor's failure to conduct routine checks on the patient's infection indicators and microbial cultures, it is difficult to determine whether the symptoms are caused by infection, disease, or CIP.
CONCLUSION
The severity of CIP and the initial dose of methylpredni-solone were observed to be significant factors affecting the outcome of CIP, while the use of antibiotics was not. These results suggest that for CIP patients, it is necessary to identify whether there is a co-infection, accurately determine the appropriate usage of antibiotics, and adopt a rational antibiotic treatment.
ACKNOWLEDGEMENTS:
This work was supported by the Scientific Research Project of Hunan Provincial Health Commission (No. 202202045392 and No. 202213054953).
ETHICAL APPROVAL:
The Ethics Committee on Biomedical Research approved this retrospective study with partial exemption from informed consent (2023KYKS NO.72).
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
MZ: Presented the ideas and was responsible for the organisation and coordination of the trial.
JLSC: Developed the trial design and wrote the original draft.
MZT: Data analysis.
MZSC: Reviewed and edited the manuscript.
All authors contributed to the management or administration of the trial and approved the final version of the manuscript to be published.
REFERENCES
- Tiu BC, Zubiri L, Iheke J, Pahalyants V, Theodosakis N, Ugwu-Dike P, et al. Real-world incidence and impact of pneumonitis in patients with lung cancer treated with immune checkpoint inhibitors: a multi-institutional cohort study. J Immunother Cancer 2022; 10(6):e004670. doi: 10.1136/jitc-2022-004670.
- Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 2017; 35(7):709-17. doi: 10.1200/JCO. 2016.68.2005.
- Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol 2016; 2(12): 1607-16. doi: 10.1001/jamaoncol.2016.2453.
- Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 2019; 16(9):563-80. doi: 10.1038/s41571-019- 0218-0.
- Chuzi S, Tavora F, Cruz M, Costa R, Chae YK, Carneiro BA, et al. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag Res 2017; 9:207-13. doi: 10. 2147/CMAR.S136818.
- Atchley WT, Alvarez C, Saxena-Beem S, Schwartz TA, Ishizawar RC, Patel KP, et al. Immune checkpoint inhibitor-related pneumonitis in lung cancer: real-world incidence, risk factors, and management practices across six health care centers in north carolina. Chest 2021; 160(2):731-42. doi: 10.1016/j.chest.2021.02.032.
- Zhai X, Zhang J, Tian Y, Li J, Jing W, Guo H, et al. The mechanism and risk factors for immune checkpoint inhibitor pneumonitis in non-small cell lung cancer patients. Cancer Biol Med 2020; 17(3):599-611. doi: 10.20892/j.issn.2095- 3941.2020.0102.
- Zhang C, Gao F, Jin S, Gao W, Chen S, Guo R. Checkpoint inhibitor pneumonitis in Chinese lung cancer patients: clinical characteristics and risk factors. Ann Palliat Med 2020; 9(6):3957-65. doi: 10.21037/apm-20-1823.
- Jiang S, Geng S, Chen Q, Zhang C, Cheng M, Yu Y, et al. Effects of concomitant antibiotics use on immune check-point inhibitor efficacy in cancer patients. Front Oncol 2022; 12:823705. doi: 10.3389/fonc.2022.823705.
- De Giglio A, Mezquita L, Auclin E, Blanc-Durand F, El-Amarti L, Caramella C, et al. Impact of intercurrent introduction of steroids on clinical outcomes in advanced non-small-cell lung cancer (NSCLC) patients under immune-checkpoint inhibitors (ICI). Cancers (Basel) 2020; 12(10):2827. doi: 10.3390/cancers12102827.
- Yan T, Yu L, Shangguan D, Li W, Liu N, Chen Y, et al. Advances in pharmacokinetics an pharmacodynamics of PD-1/PD-L1 inhibitors. Int Immunopharmacol 2023; 115: 109638. doi: 10.1016/j.intimp.2022.109638.
- Thompson JA, Schneider BJ, Brahmer J, Achufusi A, Armand P, Berkenstock MK, et al. Management of immunotherapy-related toxicities, Version 1.2022, NCCN clinical practic guidelines in oncology. J Natl Compr Canc Netw 2022; 20(4):387-405. doi: 10.6004/jnccn.2022.0020.
- Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. AnnOncol 2018; 29(Suppl 4):iv264-iv6. doi: 10.1093/annonc/mdy162.
- Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018; 36(17):1714-68. doi: 10.1200/JCO.2017.77.6385.
- Delaunay M, Prevot G, Collot S, Guilleminault L, Didier A, Mazieres J. Management of pulmonary toxicity associated with immune checkpoint inhibitors. Eur Respir Rev 2019; 28(154):190012. doi: 10.1183/16000617.0012-2019.
- Ross JA, Komoda K, Pal S, Dickter J, Salgia R, Dadwal S. Infectious complications of immune checkpoint inhibitors in solid organ malignancies. Cancer Med 2022; 11(1):21-7. doi: 10.1002/cam4.4393.
- Patel J, Crawford JM. Microbiota-regulated outcomes of Human Cancer immunotherapy via the PD-1/PD-L1 axis. Biochemistry 2018; 57(6):901-3. doi: 10.1021/acs.bio chem.7b01249.
- Routy B LCE, Derosa L, Duong CPM, Alou MT, Daille`re R, et al. Gut microbiome influences efficacy of PD-1 ebased immunotherapy against epithelial tumors. Science 2018; 359(6371):91-7. doi: 10.1126/science.aan3706.
- Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncology 2019; 5(12):1774-78. doi: 10.1001/jamaoncol.2019.2785.